LC/MS/MS Method for Determination of Antitussives
| Method: | HPLC-MS | ||||||||||||||||||||||||||||||||||||
| Matrix: | Medicine | ||||||||||||||||||||||||||||||||||||
| Application No.: | 101937 | ||||||||||||||||||||||||||||||||||||
| Componds: | Butethamate citrate, Dextromethorphan hydrobromide, Carbetapentane citrate, Oxeladin citrate, Cloperastine, hydrochloride |
||||||||||||||||||||||||||||||||||||
| Columns: | Endeavorsil C18 1.8μm , 150x2.1mm | ||||||||||||||||||||||||||||||||||||
| Cat No.: | 87004 | ||||||||||||||||||||||||||||||||||||
| Sample Pretreatment: | Samples: Stock solution of each antitussives was prepared in acetonitrile at a concentration of 1 mg/mL. Combined working solution was freshly obtained by dilution of stock solution with mobile phase. | ||||||||||||||||||||||||||||||||||||
| Conditions: | Mobile Phase: A: 0.1% formic acid in 20M ammonium formate B: 0.1% formic acid in 25/25/50(v/v/v) ethanol:methanol: acetonitrile Gradient: 10%B hold 1.5min, 10 to 30%B in 2.5min, 30 to 40%B in 2min, 40 to 50%B in 4min, 50 to 70%B in 3min, hold 4min, 70 to 10%B in 0.5min, equilibrate at 10%B for 3.5 min Scan mode: ESI+; Flow Rate: 0.2mL/min; Injection: 2µL; Temperature: 25℃ |
||||||||||||||||||||||||||||||||||||
| Publisher: | Dikma Technologies Inc. | ||||||||||||||||||||||||||||||||||||
| Keyword: | Antitussives, LC/MS/MS, Butethamate citrate, Dextromethorphan hydrobromide, Carbetapentane citrate, Oxeladin citrate, Cloperastine, hydrochloride | ||||||||||||||||||||||||||||||||||||
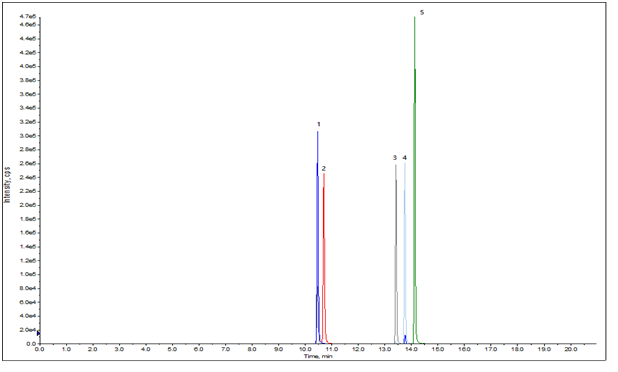
| Abstract: | These medicines have similar molecular structures, they can be well separated on DIKMA Endeavorsil C18 Columns(Cat#87004) within 15 mins, coupled with sensitive MS analysis. | ||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
USA
51 Massier Lane
Foothill Ranch, CA 92610, USA
Tel: 1-866-889-9072
Fax: 1-866-833-2653
Email: sale@dikmatech.com
Business hours: 9 AM - 5 PM PST
Canada
255 Shields Court, Unit A
Markham, ON L3R 8V2, Canada
Tel: 905-944-8066
Fax: 905-944-0181
Toll-Free:1-866-889-9072
Email: sales@dimaglass.com
Business hours: 9 AM - 5 PM EST
Asia / Pacific Area
Room 9, 5F., No.763 Wenlin Road,
Shilin District, Taipei City
111, Taiwan
Email: paulw@dikmatech.com