Ceftizoxime Sodium
| Method: | HPLC | ||||||||||||||||||
| Matrix: | Medicine | ||||||||||||||||||
| Application No.: | 101963 | ||||||||||||||||||
| Componds: | Ceftizoxime Sodium, Salicylic Acid | ||||||||||||||||||
| Columns: | Diamonsil® Plus C18 5μm 150 x 4.6 mm | ||||||||||||||||||
| Cat No.: | 99401 | ||||||||||||||||||
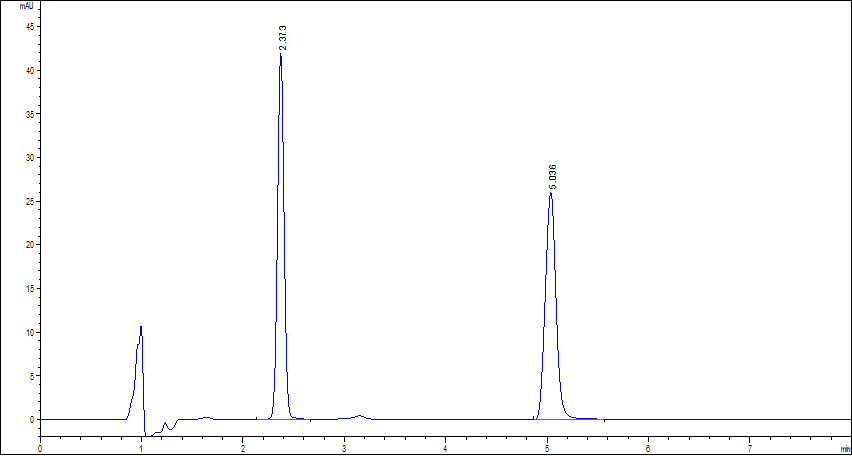
| Sample Pretreatment: | pH 7.0 buffer: Dissolve 3.63 g of monobasic potassium phosphate and 10.73 g of dibasic sodium phosphate in water to obtain 1000 mL of solution. Salicylic acid solution: Dissolve 0.6 g of salicylic acid in 5 mL of methanol and dilute with pH 7.0 Buffer to obtain 100 mL of solution. Ceftizoxime Sodium solution: Dissolve a suitable quantity of ceftizoxime Sodium, accurately weighted, in pH 7.0 Buffer to obtain a solution having a known concentration of 1 mg of ceftizoxime sodium per mL. System suitability solution: Transfer 0.2 mL of Ceftizoxime Sodium solution and 0.5 mL of Salicylic acid solution, dilute with pH 7.0 Buffer to 10 mL, and mix. This solution contains about 0.02 mg of ceftizoxime sodium per mL. |
||||||||||||||||||
| Conditions: | Mobile phase: Dissolve 1.42 g of citric acid monohydrate and 0.865 g of dibasic sodium phosphate in water to obtain 1000 mL of pH 3.6 buffer. Prepare a mixture of pH 3.6 buffer and acetonitrile (9: 1) Detector: UV 254 nm Flow rate: 1.8 mL/min Injection volume: 7 µL Column temp.: 30 ℃ |
||||||||||||||||||
| Publisher: | Dikma Technologies Inc. | ||||||||||||||||||
| Keyword: | Ceftizoxime Sodium, Salicylic Acid, HPLC, Diamonsil Plus C18 | ||||||||||||||||||
| Abstract: | Ceftizoxime Sodium is the sodium salt form of ceftizoxime and a semi-synthetic, broad-spectrum, beta-lactamase resistant, third-generation cephalosporin. The experimental conditions follow guidelines from the USP43-NF38 monograph method. | ||||||||||||||||||
|
|||||||||||||||||||
Contact Dikma:
USA
51 Massier Lane
Foothill Ranch, CA 92610, USA
Tel: 1-866-889-9072
Fax: 1-866-833-2653
Email: sale@dikmatech.com
Business hours: 9 AM - 5 PM PST
Canada
255 Shields Court, Unit A
Markham, ON L3R 8V2, Canada
Tel: 905-944-8066
Fax: 905-944-0181
Toll-Free:1-866-889-9072
Email: sales@dimaglass.com
Business hours: 9 AM - 5 PM EST
Asia / Pacific Area
Room 9, 5F., No.763 Wenlin Road,
Shilin District, Taipei City
111, Taiwan
Email: paulw@dikmatech.com