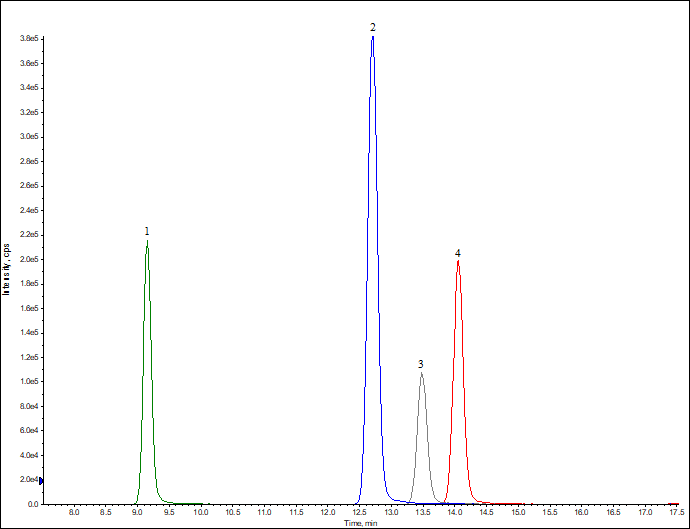
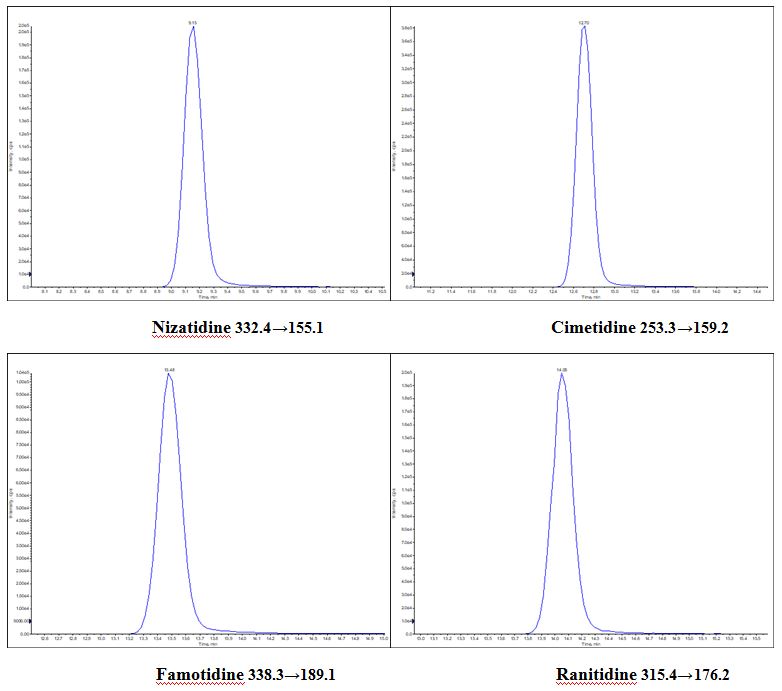
Determination of H2 Blockers by UHPLC-MSMS Method Using DIKMA Endeavorsil C18 Column
| Method: | HPLC-MS | ||||||||||||||||||||||||||||||
| Matrix: | Medicine | ||||||||||||||||||||||||||||||
| Application No.: | 101965 | ||||||||||||||||||||||||||||||
| Componds: | Nizatidine, Cimetidine, Famotidine, Ranitidine | ||||||||||||||||||||||||||||||
| Columns: | Endeavorsil C18-A 1.8μm , 100x2.1mm | ||||||||||||||||||||||||||||||
| Cat No.: | 87113 | ||||||||||||||||||||||||||||||
| Sample Pretreatment: | Standards: Individual stock solutions were prepared in methanol/acetonitrile (1/5) at a concentration of 1 mg/mL. A working solution of the mixture of all 4 drugs was obtained by dilution of the stock solution with mobile phase. | ||||||||||||||||||||||||||||||
| Conditions: | Mobile Phase: A: Ammonium Formate (pH4.7) B: methanol: acetonitrile = 4: 6 Mobile Phase A: Accurately weigh 23g of formic acid into water, and add water to make the final volume 100mL. Pipette out exactly 15mL of this solution, adjust the pH to 4.7 with 10M ammonium hydroxide. Transfer this solution into a vessel, bring up the volume to 0.5L with water. Gradient: hold 5% B for 2 min, 5 to 8% B in 13 min, 8 to 60% B in 2 min, 60 to 5% B in 1 min, equilibrate at 5% B for 5 min. Flow Rate: 0.2 mL/min Injection: 2 µL Temperature: 25 ℃ Source: ESI Ionization Mode: Positive |
||||||||||||||||||||||||||||||
| Publisher: | Dikma Technologies Inc. | ||||||||||||||||||||||||||||||
| Keyword: | Nizatidine, Cimetidine, Famotidine, Ranitidine, Endeavorsil C18-A | ||||||||||||||||||||||||||||||
| Abstract: | This method was establishd by using DIKMA Endeavorsil 1.8 µm C18-A column. With simple mobile phase and a gradient procedure, these four compounds can be eluted from the column within 15 mins. | ||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
USA
51 Massier Lane
Foothill Ranch, CA 92610, USA
Tel: 1-866-889-9072
Fax: 1-866-833-2653
Email: sale@dikmatech.com
Business hours: 9 AM - 5 PM PST
Canada
255 Shields Court, Unit A
Markham, ON L3R 8V2, Canada
Tel: 905-944-8066
Fax: 905-944-0181
Toll-Free:1-866-889-9072
Email: sales@dimaglass.com
Business hours: 9 AM - 5 PM EST
Asia / Pacific Area
Room 9, 5F., No.763 Wenlin Road,
Shilin District, Taipei City
111, Taiwan
Email: paulw@dikmatech.com